Which Statement Best Explains The Symbol [nacl]?
Which statement best explains the symbol [nacl]?. Because the formation of Na2 is an unfavorable process due to the great amount of energy required to remove the 2nd electron from the Na atom the 2nd electron would. 5844 x 002 11688 g However there are only 350 ml which is 035 liters. Metal M has only one oxidation number and forms a compound with the formula.
A The attractions between particles in solid A are stronger than those between particles in solid B. Atomic models of graphite and diamond are shown. All of the following statements are true EXCEPT.
A 002 M solution contains 002 moles per liter. Which statement best explains how the solution should be made. O Add 496 mL of 0250 M sucrose to 4000 mL of water to get 4000 mL of 00310 M sucrose.
Atoms Are Most Stable With Eight Electrons In Their Valence Shell And The Electron Configuration Of A Noble Gas. O Add 496 mL of 00310 M sucrose to 3504 ml of water to get 4000 mL of 0250 M sucrose. 0409 g As stated 1 mole of NaCl weighs 5844 g.
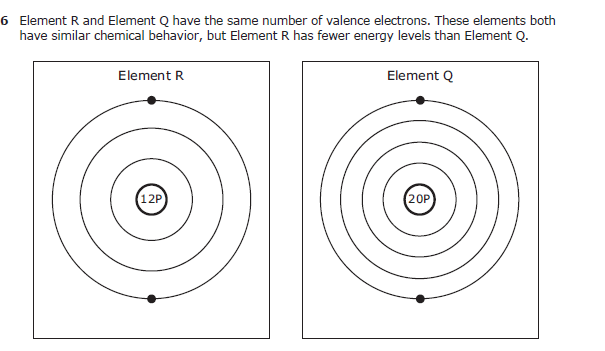
Sodium is the major cation of extracellular fluid and functions principally in the control of water distribution fluid and electrolyte balance and osmotic pressure of body fluids. A molecule of methanol is composed of carbon atoms connected to 3 hydrogen atoms and a hydroxide group. Which Statement Best Explains The Basis For The Octet Rule.
Click here to get an answer to your question Which of the following chemical symbols represents the element sodium. C The mass of particles in solid A is greater than the mass of particles in solid B. The chemical formula of sodium chloride is NaCl and its molar mass is 5844 gmol.
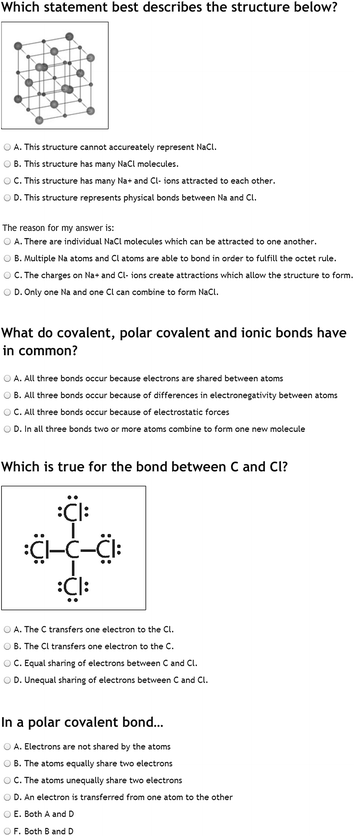
Atoms Are Most Stable With. It is an ionic compound consisting of a sodium cation Na and a chloride anion Cl-Solid NaCl has a crystalline structure in which each Na ion is surrounded by six chloride ions in an octahedral geometry.
Which of the following statements are not correct.
Atoms Are Most Stable When They Have Eight Bonds. 0409 g As stated 1 mole of NaCl weighs 5844 g. Which of the following statements best explains why. One of the X-atoms is missing from one of the faces in the unit cell. A 002 M solution contains 002 moles per liter. Hence 1 litre would contain. Ii In canonical structures there is a difference in the arrangement of atoms. 5844 x 002 11688 g However there are only 350 ml which is 035 liters. In the first video clip the sodium flares up almost immediately upon reaction with the water and burns out quickly.
The chemical formula of sodium chloride is NaCl and its molar mass is 5844 gmol. Atoms Are Most Stable With. Explain why solid sodium chloride does not conduct electricity but molten sodium chloride does. Which of the following statements are not correct. A molecule of methanol is composed of carbon atoms connected to 3 hydrogen atoms and a hydroxide group. Sodium chloride is present in the sea and ocean waters giving. It is an ionic compound consisting of a sodium cation Na and a chloride anion Cl-Solid NaCl has a crystalline structure in which each Na ion is surrounded by six chloride ions in an octahedral geometry.





























Post a Comment for "Which Statement Best Explains The Symbol [nacl]?"